Potassium Dichromate: K2Cr2O7
Chem Avenue CBSE-12, Inorganic Chemistry #CBSE, #d and f block elements, #Inorganic chemistry, #NCERT 0
K2Cr2O7
Uses:
1. In leather industry
2. Oxidizing agent for the preparation of azo compounds in organic chemistry
Preparation:
This can be prepared from Chromite ore (FeCr2O4) in three steps.
Step1: fusion of chromite ore (FeCr2O4) with sodium or potassium carbonate in free access of air(O2). This results Yellow solution of sodium chromate(Na2CrO4).
4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
Step2:Acidification of the resulting yellow chromate solution with H2SO4 gives orange crystals of sodium dichromate dihydrate(Na2Cr2O7.2H2O)
2Na2CrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
Step3:Potassium dichromate is less soluble than sodium dichromate. Therefore, treating of sodium dichromate(Na2Cr2O7) with KCl gives orange crystals of K2Cr2O7.
Na2Cr2O4 + 2KCl → K2Cr2O7 + 2NaCl
Interconversion between chromate(CrO42−) and dichromate(Cr2O72−)
As the pH changes Both yellow chromate (CrO42−) and orange dichromate (Cr2O72−) interconvert each other. (Remember: BOY: Basic Medium ⟹ Orange to Yellow). In acidic medium yellow chromate changes to orange dichromate and in basic medium orange dichromate changes to yellow.
2CrO42−.(Yellow) + 2H+ → Cr2O72− (Orange)+ H2O
Cr2O72−(Orange) + 2OH− → 2CrO42−(Yellow) + H2O
Structure of Chromate and Dichromate
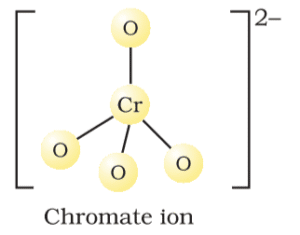
Structure of Chromate:
In chromate Cr(VI) is tetrahedrally surrounded four oxygen atoms.
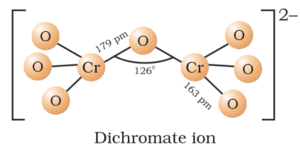
Structure of Dichromate:
In dichromate ion, two tetrahedral CrO4 units share one oxygen atom with Cr-O-Cr bond angle 126o.
Chemical properties
Sodium and potassium chromates are strong oxidizing agents. Sodium dichromate used as oxidizing agent in organic chemistry and K2Cr2O7 used as primary standard in volumetric analysis.
Oxidizing properties of K2Cr2O7 .
In acidic medium, Cr2O72− reduces to Cr3+ and it oxidizes the following:
Iodides to Iodine: I− → I2
Ferrous to Ferric: Fe2+ → Fe3+
Tin(II) to Tin(IV): Sn2+ → Sn4+
Sulfide to Sulfur: H2S → S
Reduction of Cr2O72− in acidic medium: 14H+ + Cr2O72− + 6e− → 2Cr3+ + 7H2O (Eo = 1.33V). Addition of this half reaction to the above four half reactions results the following balanced redox reactions.
1. 14H+ + Cr2O72− + 6I− → 2Cr3+ + 3I2 + 7H2O
2. 14H+ + Cr2O72− + 6Fe2+ → 2Cr3+ + 6Fe3+ + 7H2O
3. 14H+ + Cr2O72− + 3Sn2+ → 2Cr3+ + 3Sn4+ + 7H2O
4. 8H+ + Cr2O72− + 3H2S→ 2Cr3+ + 3S + 7H2O