Lithium Aluminum Hydride, LiAlH4
LiAlH4(Lithium aluminum hydride) is a strong reducing agent for the organic functional group transformations. LiAlH4can be synthesized from LiH and AlCl3 in ether solvent as follows.
3LiH + AlCl3 ⟶ LiAlH4 + 3LiCl
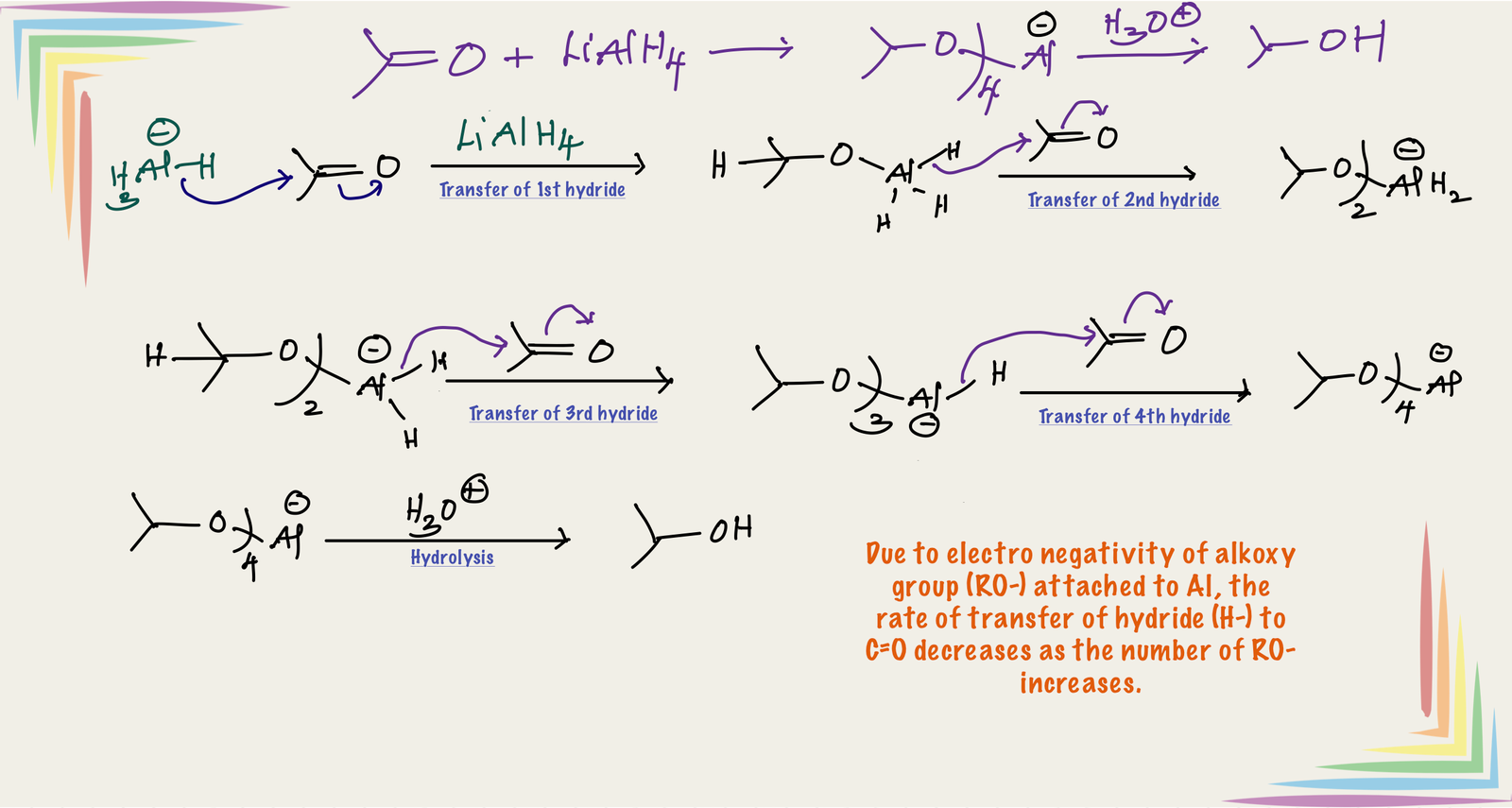
Lithium aluminum hydride acts as a reducing agent through the transfer of hydride from LiAlH4 to the electrophilic center, like carbon of C=O in carbonyl compounds. All hydrogen of LiAlH4 transfer to stepwise manner as shown in the below mechanism. After transfer of one hydrogen forms alkoxy substituted aluminum hydride, from which transfer of hydrogen rate is slow. Therefore, alkoxy substituted lithium aluminum hydrides are relatively mild reducing and more selective than LiAlH4 itself.
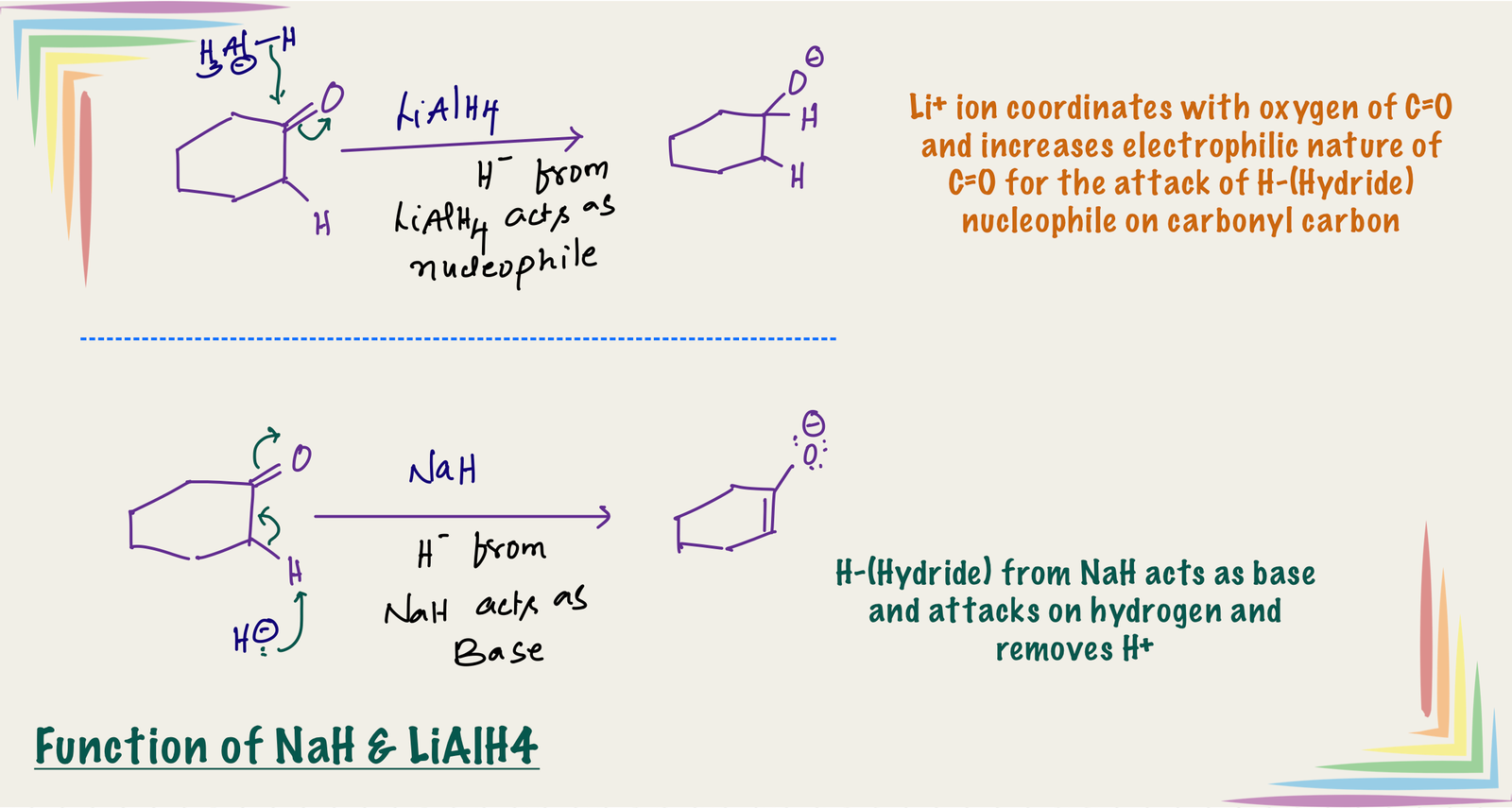
Hydride of LiAlH4 acts as nucleophile and that of NaH acts as base
H–(hydride) can acts as nucleophile and base. LiAlH4 is hydride donor and hydride (H–) is highly reactive in solutions and it does not exist independently in solution. In LiAlH4 hydride(H-) is covalently bonded with less reactive aluminum, therefore it carries a partial negative charge, which makes it less basic [Unlike in NaH, where is carries a -1 charge].
LiAlH4 versus NaH:
In NaH, H– is a strong basic and weak nucleophile where as H- in LiAlH4 acts as nucleophilic rather than basic. [Nucleophile attacks on carbon and replaces a group from carbon in nucleophilic substitutions but base attacks on hydrogen which is relatively acidic]
Solvents: Solvents which are suitable for LiAlH4 are ethers. H2O and ROH cannot be used as solvents because LiAlH4react with these solvents and produces H2 gas. Common solvents used for LiAlH4 reductions are diethyl ether and THF (tetrahydro furan).
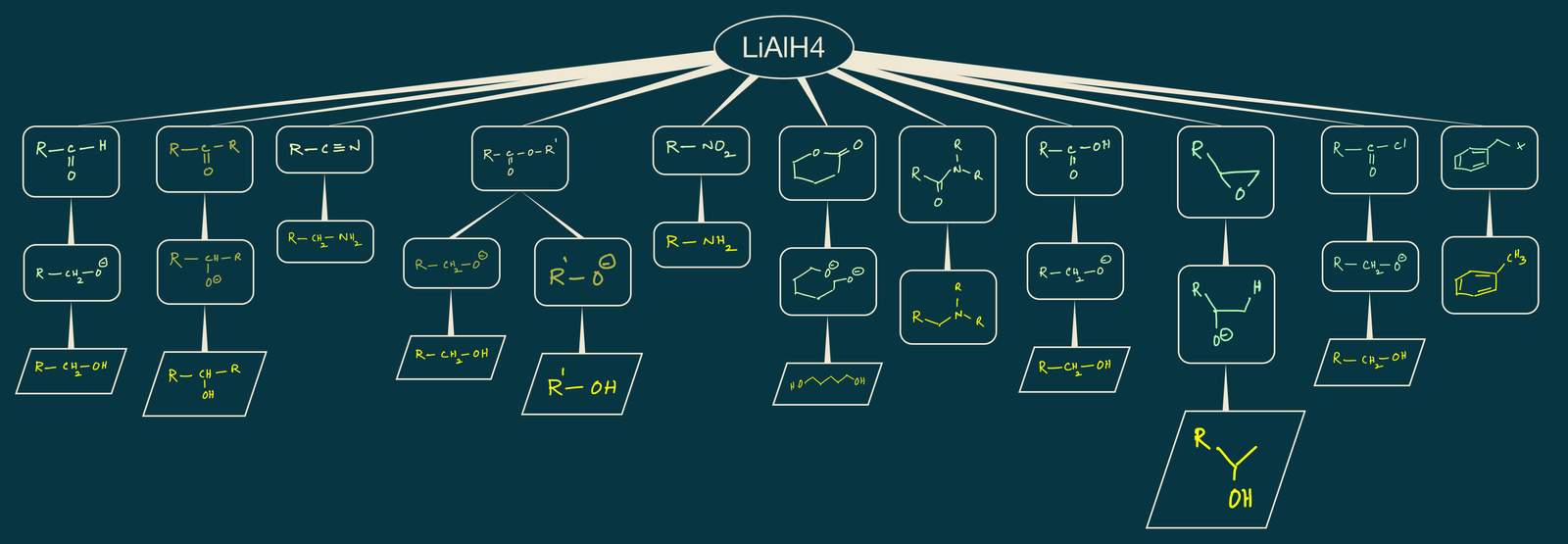
Reactions of LiAlH4:
Lithium aluminum hydride is a strong reduces and it reduces almost all functional groups. It reduces aldehydes, ketones, carboxylic acids, acid anhydrides, acid chlorides, esters, lactones, amides, lactams, carbamates, and cyanides etc.,
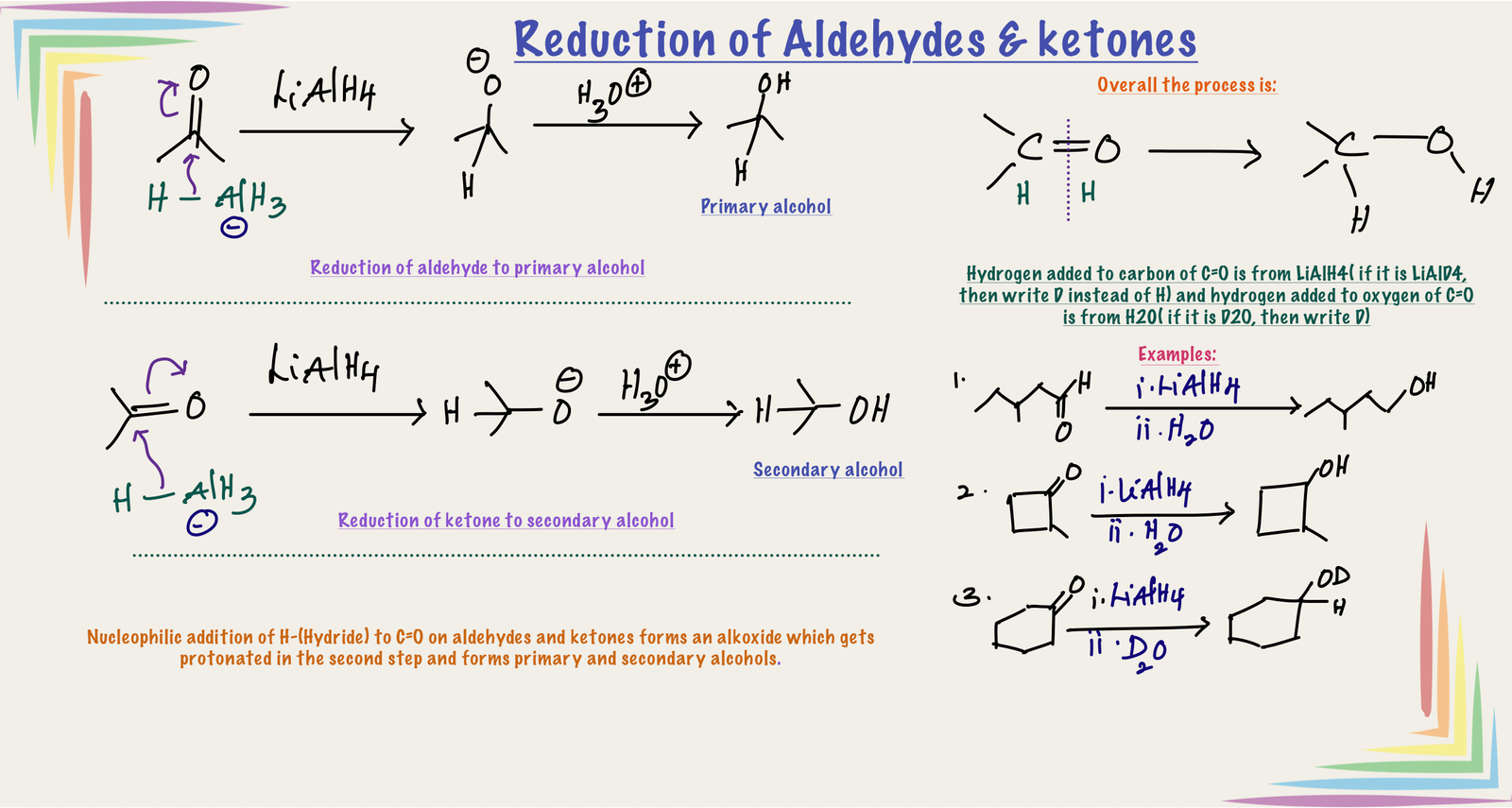
Reduction of aldehydes and ketones:
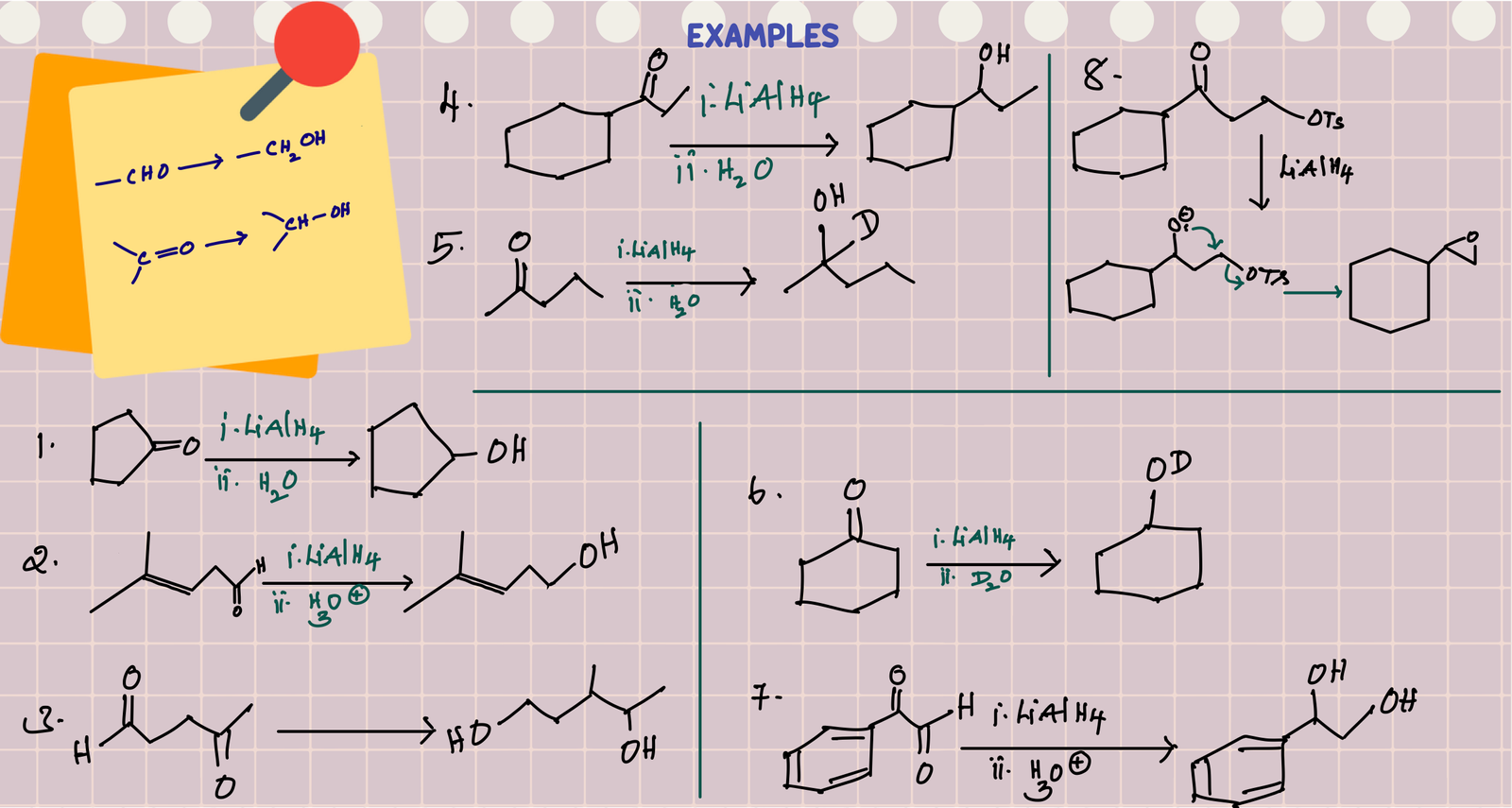
Reduction of aldehydes and ketones by LiAlH4 followed by acidic hydrolysis gives alcohols as products by the nucleophilic addition of hydride from LiAlH4 to the carbonyl carbon of C=O group. Coordination lithium ion with oxygen of C=O makes carbonyl carbon more electrophilic which promotes the attack of nucleophile(H-) on C=O.
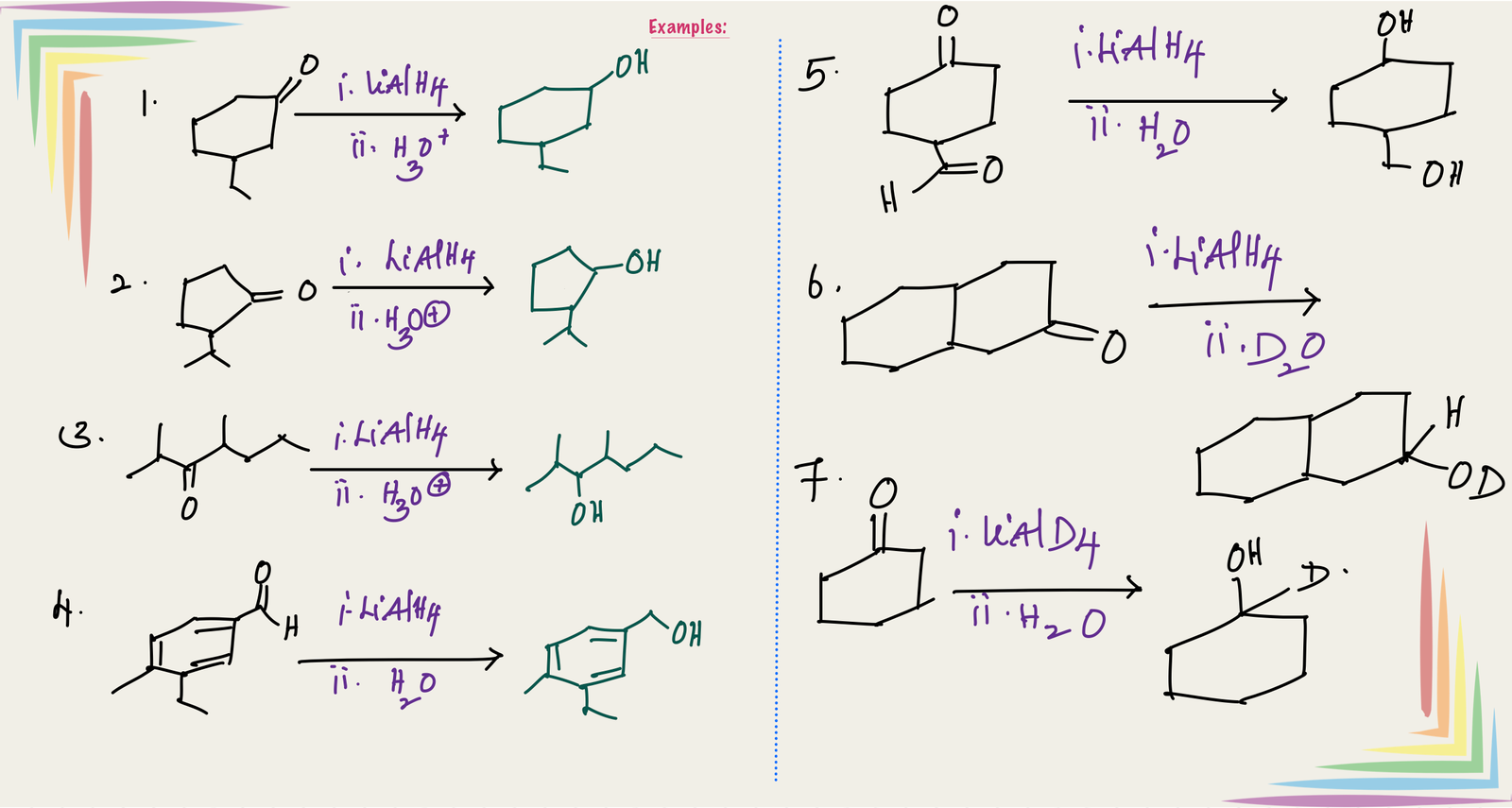
Reduction of aldehyde gives primary alcohol(1o R-OH) and reduction of ketones gives secondary alcohol (2o R-OH). Overall reaction involves addition of two hydrogens at C=O, one hydrogen to carbon (Source of this hydrogen is hydride donor, LiAlH4. If we use LiAlD4, then add D(deuterium)) and the other hydrogen to oxygen of C=O (Source of this hydrogen is H2O. If we use D2O, then add D)
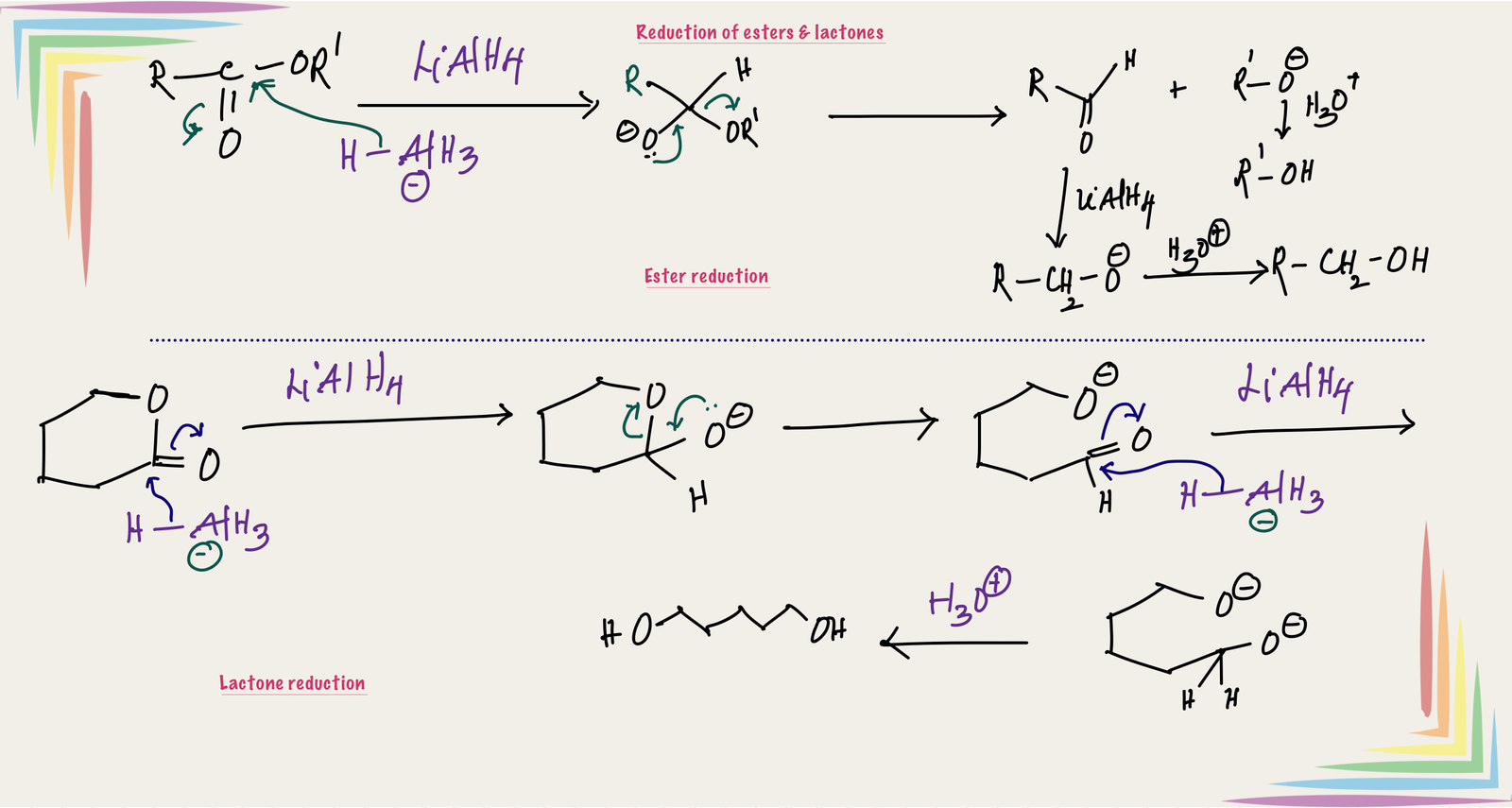
Reduction of esters:
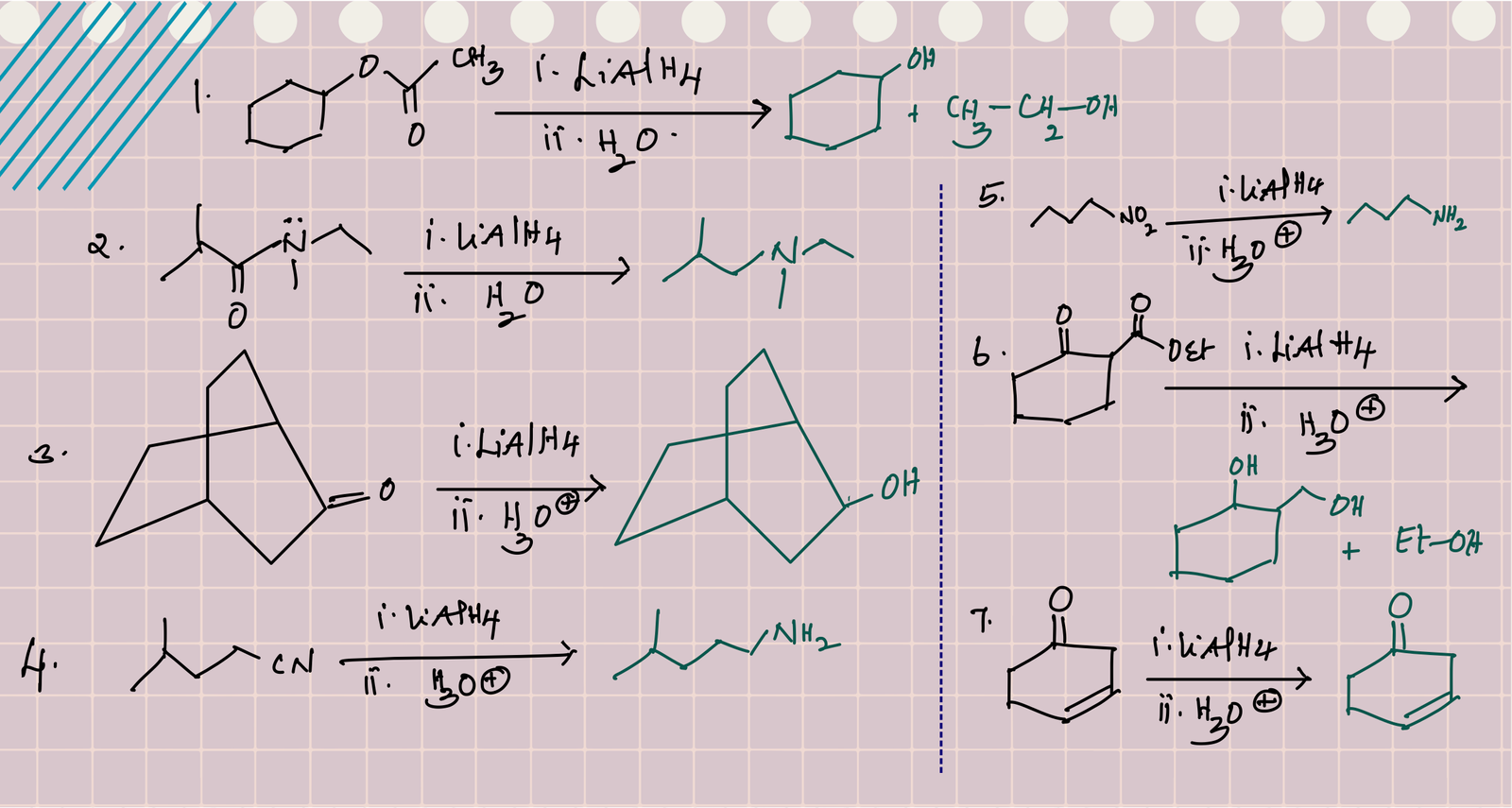
Nucleophilic addition of hydride to the C=O of ester forms a tetrahedral intermediate, which is unstable due to the presence of leaving group (RO) at tetrahedral carbon. Therefore, this tetrahedral intermediate collapse to aldehyde with the loss of RO- (this also protonates to alcohol during acidic work-up at the end). The resulting aldehyde further reduces to primary alcohol. Therefore, reduction of ester gives mixture of two alcohols.
Reduction of lactones (cyclic esters):
Reduction of lactone also follows the same mechanism of esters, and the difference is the leaving group is part of the molecule and gives diol after hydrolysis.
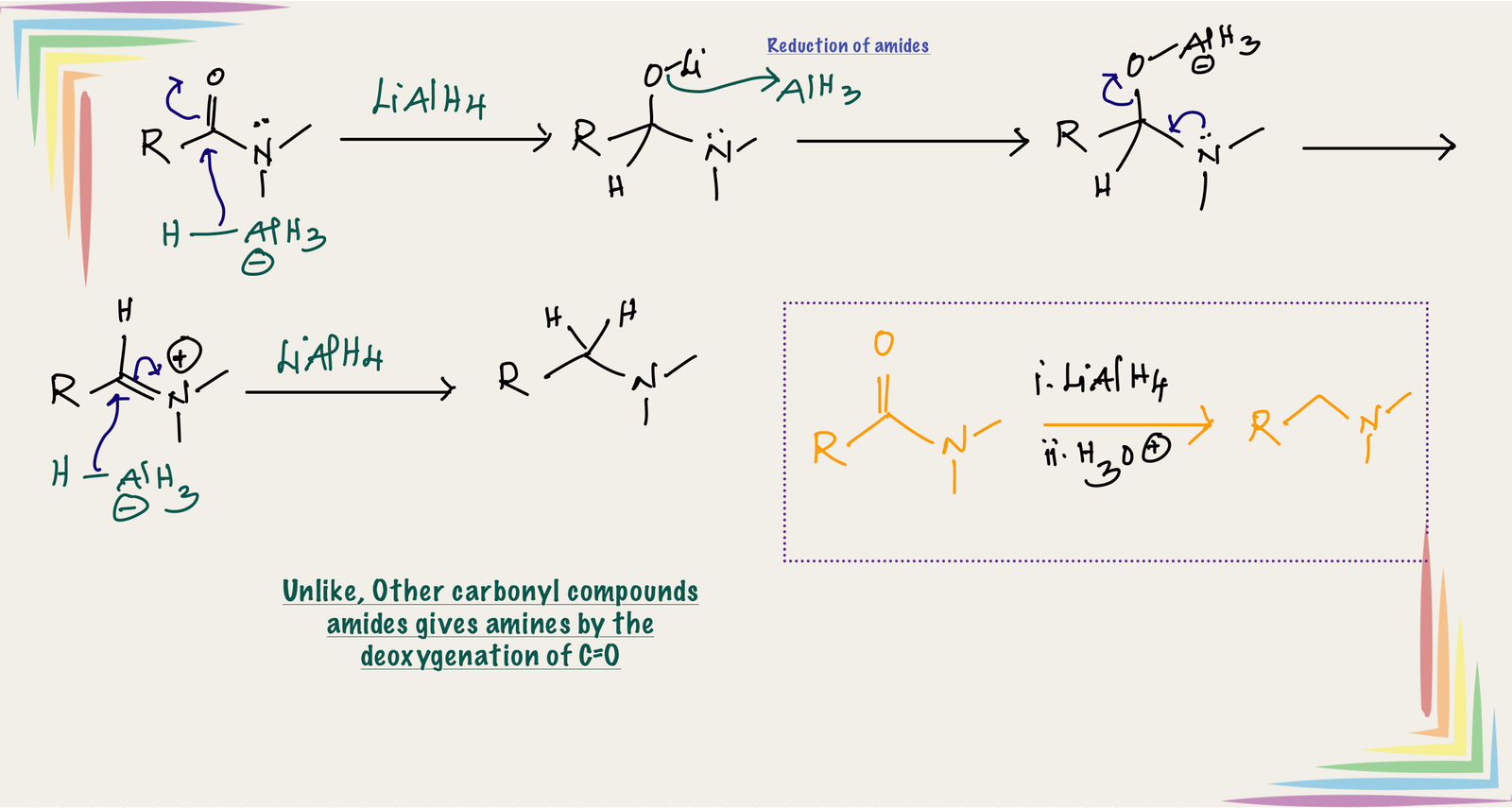
Reduction of amides:
Unlike other carbonyl derivatives, reduction of amide involves deoxygenation of C=O and forms amines as product.
Reduction of carboxylic acids: Carboxylic acid reduces to primary alcohol with LiAlH4.
RCOOH ⟶ RCH2OH
RCOOH forms RCOO– with LiAlH4 and gives hydrogen gas. Therefore, the better alternative is the conversion of acid to ester and then reduction with LiAlH4.
Reduction of alkyl halides:
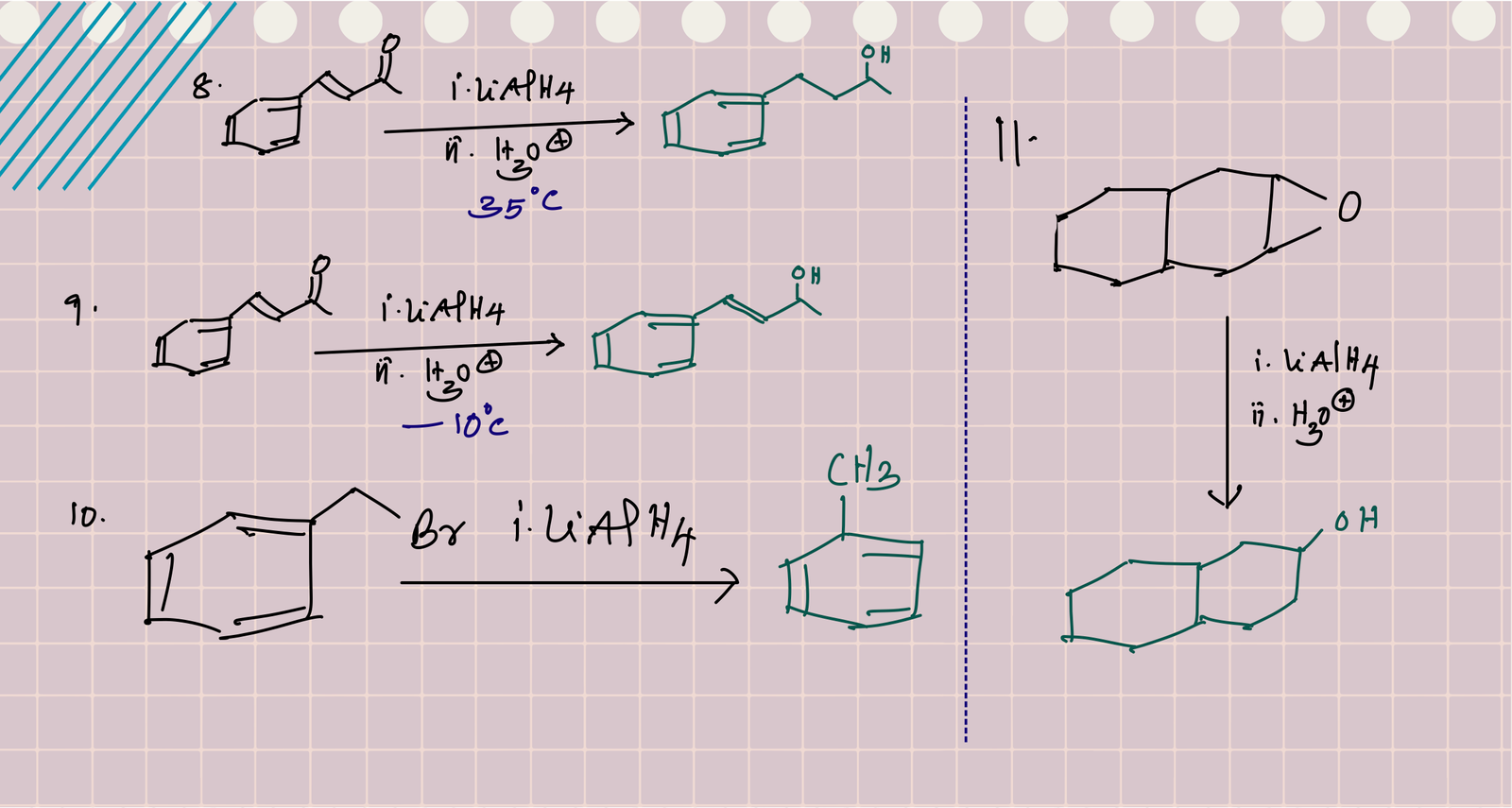
Reduction of alkyl halide forms alkanes and the process is known as hydrogenolysis because it cleaves C – X bond with hydrogens. Benzylic halides are relatively easy to reduce with LiAlH4 than aliphatic alkyl halides. Simple haloarenes do not undergo reduction with LiAlH4.
Reduction of epoxides:
Hydride (H-) attacks on the epoxide from the less hindered side and opens the ring. The resulting alkoxide on protonation gives alcohol.
Reduction of cyanides(nitriles):
Reduction of cyanides with LiAlH4 followed by acidic hydrolysis gives primary amines.
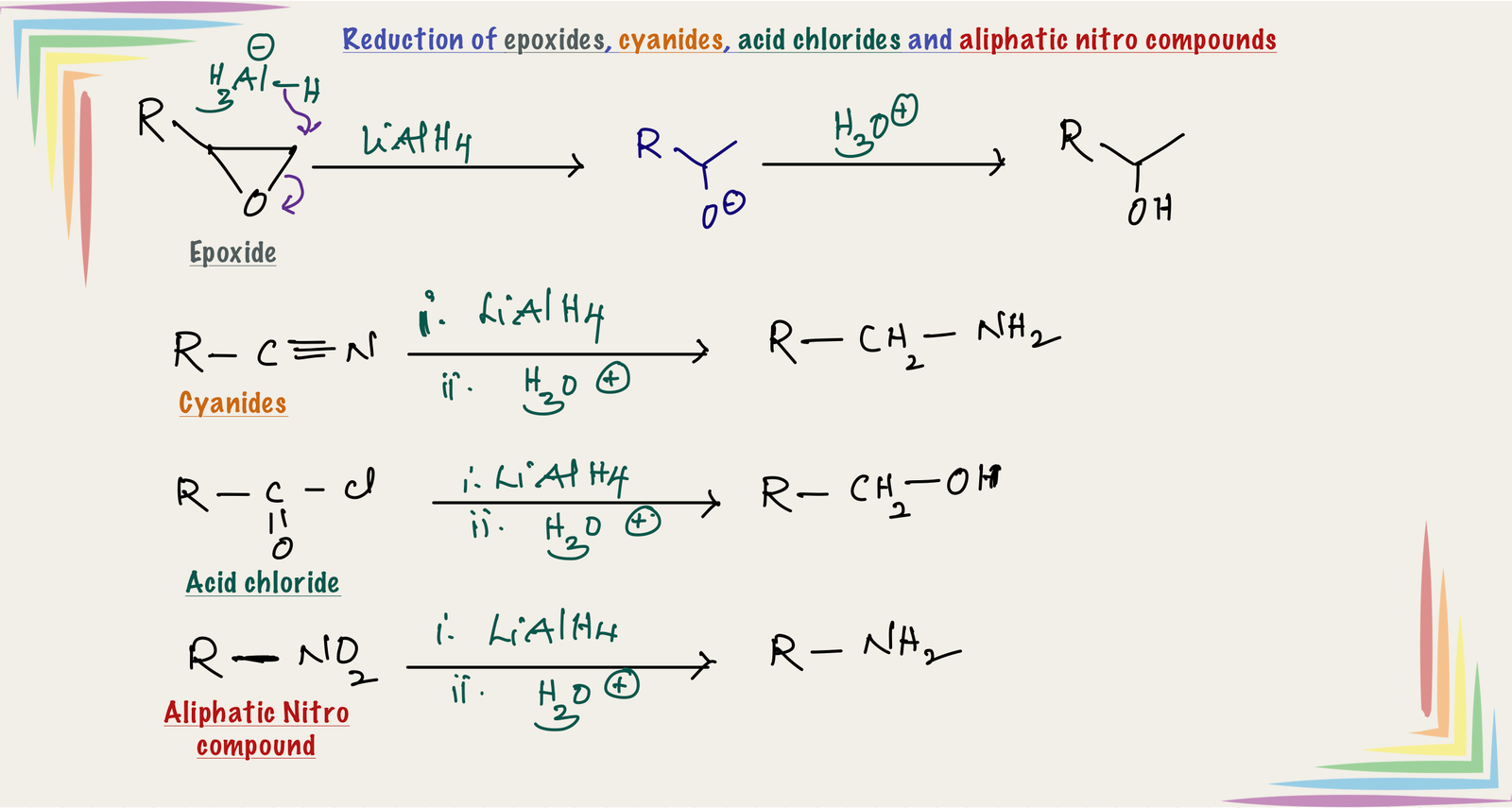
REACTIONS OF LiAlH4