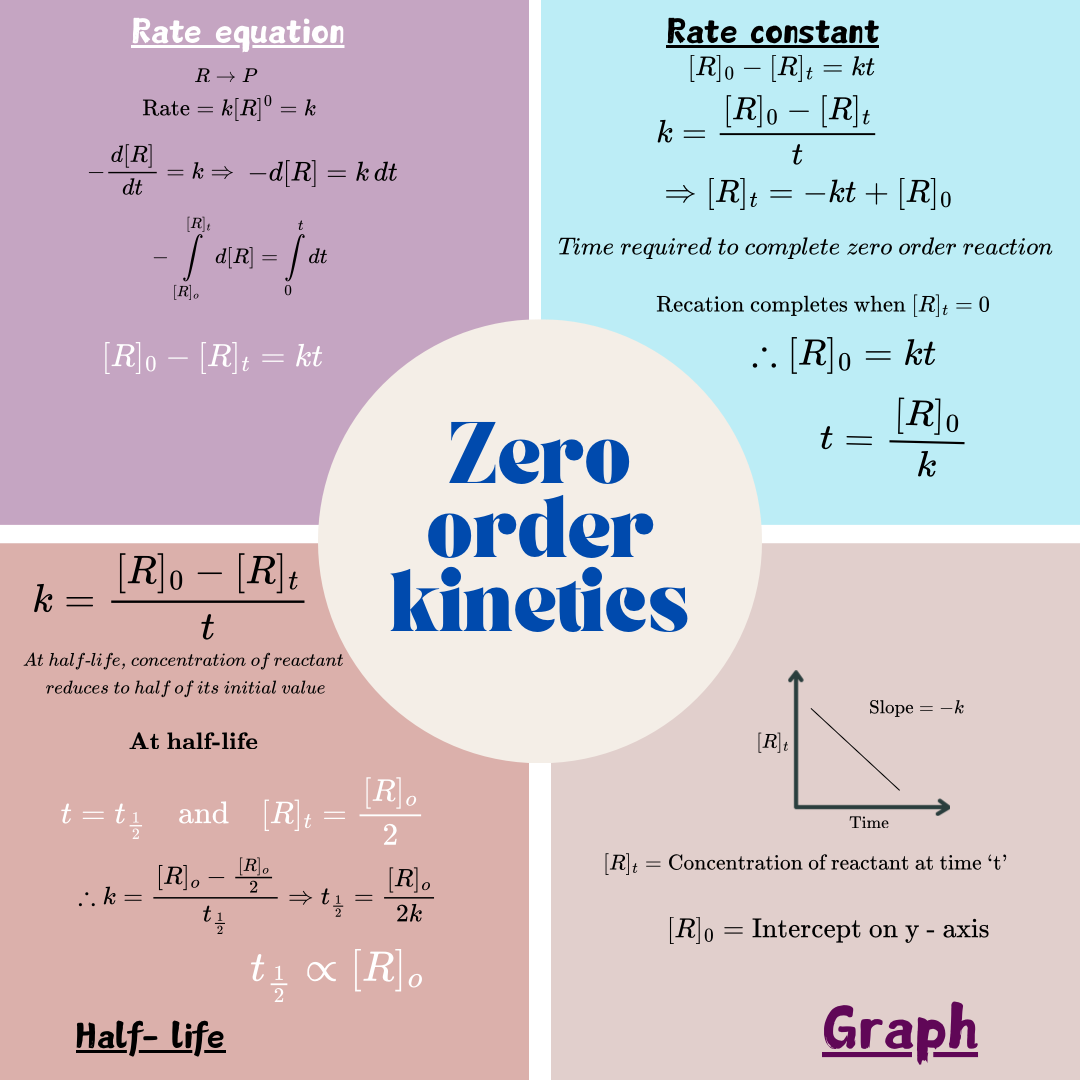
Zero order kinetics
Chemical reactions whose rate is independent on concentration of reactant are known as zero order chemical reactions. Example: Decomposition of AgBr. Half-life of zero order reactions is directly proportional to initial concentration of the reactant. Plot of concentration of reactant at time ‘t’ on y-axis and time ‘t’ on x-axis gives a straight line with slope ‘-k’ and y-intercept [R]0